I. Definition
New Food Ingredients are substances
* Not listed on national food safety standards (GB 2760 Standard for the use of Food Additives and GB 14880 Standard for the use of Food Nutrition Fortifiers)
* Not listed on announcements, issued by National Health Commission, of food additives allowed to be used
* with a extended scope of application or an increased dose
II. Timeline
It takes around 1-3 years for the application of new food additives;
It takes around 6-12 months for the application of an extended scope of application or an increased dose;
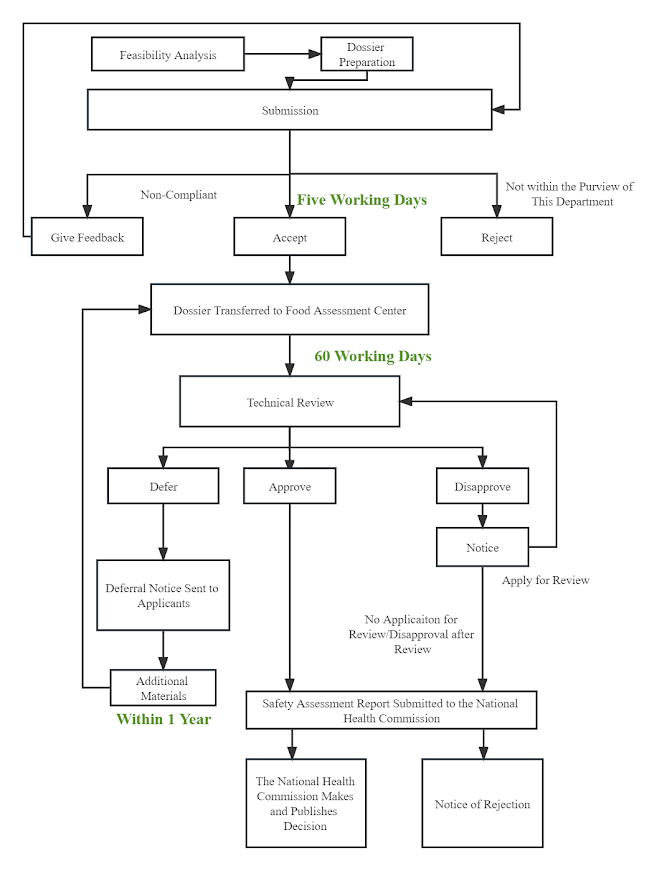
III. Process
IV. Dossier
Documents | New Varieties | Additive with an Extended Scope or an Increased Dose | |
Flavoring | Others | ||
(1) Application Form | √ | √ | √ |
(2) Name, Function, Dose, Scope of Application | √ | √ | √ |
(3) Substantiation for Technical Necessity and functions of this substance | ⅹ | √ | √ |
(4) Quality Specification, Manufacturing Process, Inspection Methods, Detecting Methods | √ | √ | √ |
(5) Safety Assessment Report | √ | √ | ⅹ |
(6) Labeling and Instructions | √ | √ | √ |
(7) Supporting Documents to Prove that the Substance is Allowed to be Produced and Used in other Countries/International Organizations | √ | √ | √ |
(8) Supporting Documents Issued by Relevant Institution or Organization to Prove that the Substance is Allowed to be Produced/Sold in the Exporting Country(Region) | √Only Applicable to Imported New Varieties | ||
(9) Supporting Documents of Examination or Accreditation of the Manufacturer Issued by the Relevant Institution or Organization in the Country (region) where the Manufacturer is Located | √Only Applicable to Imported New Varieties | ||
Note:“√” means “require”,“ⅹ”means “not require”。
V. Our Services
* Feasibility Analysis
* Application for New Food Additives
* Testings
* Other Tailored Services






0 Comments