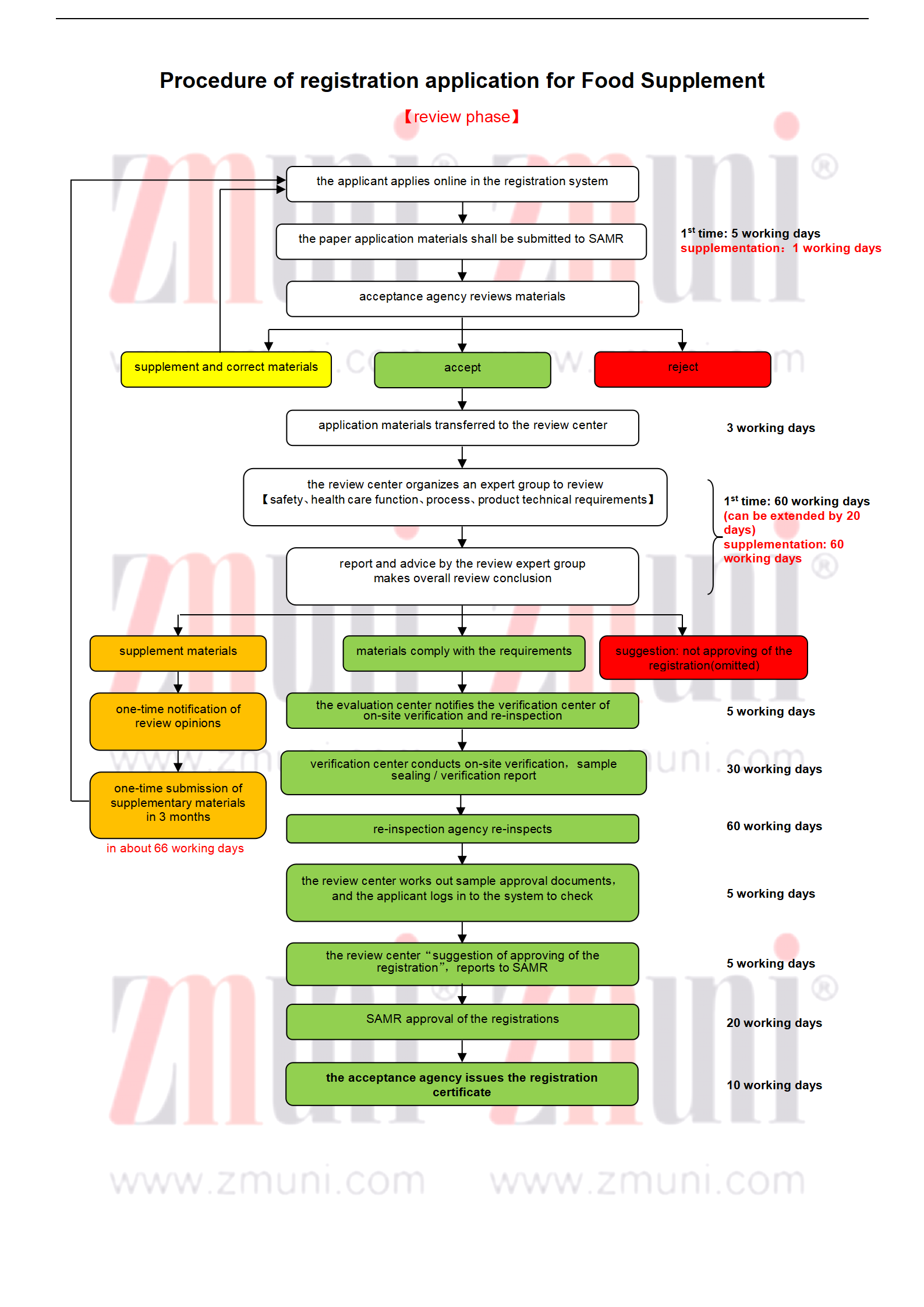
Explanation:
* Safety demonstration report and health care function demonstration report: The difficulty level and the time mainly depend on the formula, such as raw materials and their quantity, health care function and literature research results.
* Production process research report: Domestic registration depends on the actual situation of the applicant. The complete research may take more time, for example it requires the purchase/test of raw materials and auxiliary materials, process research, quality research. Imported registration can use self-inspection report and inspection report proving that the products have been produced in on a large scale more than 10 batches to replace, which takes less time.
* Registration inspection: The time mainly depends on the test method of the nominal ingredients and the type of functionality trial.
*Imported registration: Overseas applicant shall designate its branch within China or authorize the main company within China to submit the application and act as a Chinese contact.
————————————







0 Comments